The lecture hall was dark and the only faint glow was the low-lit lamp of the podium and the screen behind. Somewhere during the lecture, a hesitant hand went up in the far right corner of the room. It caught my eye and I hesitated in my delivery, then stopped and acknowledged the request.
Read In New Wide Format (or Internet Explorer Users)
Read In New Wide Format (or Internet Explorer Users)
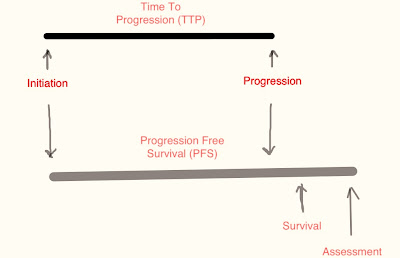
“I don’t seem to understand the difference between “Time to Progression” and “Progression Free Survival?”
“Well, they are pretty much one and the same.” I remarked then added, “One determines the end point of death from any cause (PFS) and the other only concerns with the disease progression as in (TTP) There are however several scientific publications that muddy the waters by using them interchangeably.”
“But then that would confuse a meta-analysis if those studies were compared?”
“Exactly! Good question.”
Saad and Katz state this definition in their paper:
Progression-free survival (PFS) is defined as the time elapsed between treatment initiation and tumor progression or death from any cause, with censoring of patients who are lost to follow-up. Many recent trials have used PFS or time to tumor progression (TTP) as the primary end points, with TTP theoretically differing from PFS in that the event of interest is only disease progression Both PFS and TTP have traditionally been considered as surrogate end points for OS, as far as the drug approval process is concerned… http://annonc.oxfordjournals.org/content/20/3/460.full
And thus followed a discussion that eclipsed the lecture and brought everyone to the edge of their seat. The questioner was none other than a very bright, very young nursing student. The more the discussion ensued the more her intellect blossomed before everyone’s eyes. Yes she was special. The entire episode ended after the hour had elapsed and the lecture hall lights had been turned on, but the memory lingers. Such intelligence makes for sweet memories.
And while the whole premise of PFS was and remains in doubt because of the arbitrariness of the combined data, the balm of understanding was far from comforting even for me.
To answer the progression free survival or PFS question, one might have to take on at least two issues. One is what is the importance of such a designation, in terms of new drug approvals and two, how does it impact patient care and costs in the longer term.
Lets address the issue of the makeup of the PFS and both the answers will come tumbling down like Alice falling into the rabbit hole to find more and more curious things in the math of medicine.
Dissecting the terminology, it is quite self-expressive, isn’t it? PFS determines the time period between initiation of any treatment and the progression of the disease to death. The concept being: If you get treatment A on Date 1, and disease stays stable for a duration X months, until the patient passes away from any cause, the PFS is X months. This is simple and quite straightforward, or so it seems. The implication is that the disease was kept in abeyance for a certain period of time longer than that it would with the standard therapy.
Now here are some interesting tidbits and the raw meat of the details.
Starting any study one accrues patients. The accrual rate is incremental at first and then reaches a steady state, correct? In other words if you are planning to accrue 100 patients, you are not going to get them all on the same day, are you? No! Certainly not! So there are calculations to allow for the initial accrual state and steady state to determines the PFS for each.
So far so good, you say? But here is a minor wrench, the time period allocated to the steady state is not only based on accrual but on the consideration of when the last accrual site was opened and when the public became aware of the study itself. There lies a python in that basket of surprises, if you wish to take time and think through that. What has the public awareness got to do with any of this? I really don’t have a clue. But the movers and shakers do.
So far so good, you say? But here is a minor wrench, the time period allocated to the steady state is not only based on accrual but on the consideration of when the last accrual site was opened and when the public became aware of the study itself. There lies a python in that basket of surprises, if you wish to take time and think through that. What has the public awareness got to do with any of this? I really don’t have a clue. But the movers and shakers do.
Let me inject a minor bit of information in regards to what is considered progression of a disease. The EORTC has determined a fresher guidelines for Response Evaluation Criteria or RECIST 1.2 and here by definition a disease increment of 5 mm or more, gain in size by 20% on 2-3 Dimensions of appearance of a single or multiple new site of metastasis via tangible diagnostic procedure, such as CT scan, MRI and on occasion X-RAy and clinical examination is required. All valid and true. They also mention that the thickness of the slice of 5-mm cuts as the bare minimum needed for true and verifiable reference.
This inclusion is important since a 10-mm slice may miss the lesion completely or cut through it at one end or another and thereby give a false reference to the increment or decrement of the actual size of the lesion. Of course, the contrast material used in the determination must remain constant as differing materials or none at all will give different results. (The image below copied from the article listed below for demonstration purposes only: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1435346/)
This inclusion is important since a 10-mm slice may miss the lesion completely or cut through it at one end or another and thereby give a false reference to the increment or decrement of the actual size of the lesion. Of course, the contrast material used in the determination must remain constant as differing materials or none at all will give different results. (The image below copied from the article listed below for demonstration purposes only: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1435346/)
For more read the .pdf file below:
Ok so we move on to the next level down the hole. Say one patient had a progression free disease period of 4.5 months and the second patient had a progression free period of 5.4 months, contrary to your thinking the result is not a mean, average or median. It is based on the time to assess the disease which if it is every two months then the progression free survival will be logged in as, you guessed it, 6 months or if it is every 12-weeks then it would be 7 months. Great! You say, but, isn’t that inflationary in at its basic level? So the question then arises, who is that PFS really serving, the patient or the experimenter? You make the choice.
The following are the real world calculations:
Here Ceil is the minimum integer greater than or equal to this argument.
(4.5/2)*2=3*2=6 (The calendar time of PFS is PFS plus the enrollment time)
No we are not over yet. After the accrual is complete and such a PFS has been calculated, it is then compared to the standard arm, which may be standard therapy, placebo or whatever has been deemed an appropriate measure of comparison. Those numbers are then grated by the peeler and put into the juicer of statistics and a number is arrived at which is placed on a Normal Distribution Curve and if it lies within 2-Standard deviations or a Confidence Interval of 95% that number is published (The 5% outliers need not apply). If therefore the number (X-Months) is higher than the standard therapy survival number then the treatment is considered to afford some benefit to the patient. YAY! Or just yay.
So supposing that the PFS is higher by four months (arbitrary figure) and there is an 85% probability of its validation then, my dear friends as it may be obvious there the full descriptive disclaimer would go something like this: There is a 80% chance that the PFS is 4-months better than standard therapy and falls within the Confidence Interval of 95% (CI 95%). You see! (There are two (exactly 2) percentages feeding off each other. Below is an excerpt from the PFS calculation model citing the notorious p-value.
So supposing that the PFS is higher by four months (arbitrary figure) and there is an 85% probability of its validation then, my dear friends as it may be obvious there the full descriptive disclaimer would go something like this: There is a 80% chance that the PFS is 4-months better than standard therapy and falls within the Confidence Interval of 95% (CI 95%). You see! (There are two (exactly 2) percentages feeding off each other. Below is an excerpt from the PFS calculation model citing the notorious p-value.
If you really want to dwell into the meat and potatoes all by yourself and leave this corned beef sandwich alone, please read: The FDA Guidance at various levels of discussion indicates the issues of bias in this disease assessment format:
The data is sorted by the calendar time of PFS in each trial and the calendar time to observe the pre-specified number of events is determined. Patients enrolled after this time point are eliminated in each trial. For the remaining patients, the PFS observed after this cut-off time is censored at the cut-off time. The resulting data is used to calculate the two-sided logrank p-value and the observed median PFS for each trial using the LIFETEST Procedure. The estimated power is the proportion of the number of trials with p-values less than or equal to the significance level. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071590.pdf
But even though you haven’t yet walked into the quicksand of thinking, this will really blow “your ever lovin’ mind. “ So lets say that the Proposed therapy A has shown a better PFS then the standard therapy, here is the kicker, who is to say that this has anything to do with the therapy itself but everything to do with the biology of the disease.
In other words if the number of patients are stacked in one arm of the study with a slow growing tumor against a faster growing version of the same tumor with similar stage etc. wouldn’t you get the higher PFS just by virtue of the tumor biology? Sure you would. The slow growing one would grow slowly and therefore manifest progression later. That question is answered with a, well, the computer designates the patient to the arm independent of the Principal Investigator’s knowledge. That may be so, but the bias is built in absent real information.
In other words if the number of patients are stacked in one arm of the study with a slow growing tumor against a faster growing version of the same tumor with similar stage etc. wouldn’t you get the higher PFS just by virtue of the tumor biology? Sure you would. The slow growing one would grow slowly and therefore manifest progression later. That question is answered with a, well, the computer designates the patient to the arm independent of the Principal Investigator’s knowledge. That may be so, but the bias is built in absent real information.
That is why different studies show wide disparities when comparing the same therapy choices and very few institutions or experimenters are willing to redo the study. Additionally, and now this might throw you off the curb completely, different ethnic groups have differing mechanisms to counter disease within their bodies. Yes, you read that correctly, we are after all humans but given the traits of the Transposons (those jumping genes) that manipulate the epigenetics of the DNA and suspend or accelerate certain genes, the ability to fight disease varies based on environmental and natural selective influences.
You can read about Transposons here if so inclined: http://jedismedicine.blogspot.com/search?q=transposons
The experimenters, however not willing to give up data, use any such study as a steppingstone to add another layer to the complexity, using the study as the base model. They would rather concur with prejudice then revalidate the original.
There are arguments in favor of PFS though. Huh? You shrug. Why can’t he make up his mind? Ok, in full disclosure there is a minute bit of importance, PFS noted as a function of TTP is a good indicator for first progression of disease based on high yield diagnostic procedures (e.g. CT scans, MRI and MRI/PET fusion studies). In other words using sharp diagnostic criteria the time to progression information can be of significance, but not in the sense it is being used. Needless to say, the FDA and the pharmaceutical industry use these criteria for drug approval. And you wonder why they don’t work their magic in the real world. Oh well!
Now why, I ask don’t we just go back to using the time-honored measure of absolute patient survival. Although there are some who argue to the contrary and there always are some, like the small gremlin that annoys the hell out of you when it takes a seat and grumbles at everything said in your brain and finds a contrarian’s point of view to all. The problem is expediency. TTP and to some extant PFS is faster route to show “benefit” and gain approval by the FDA.
But that can be good, you say. Yes, true, but the OS route is more deliberate and a comparator of apples and apples. The measure of determining this would be in a Randomized Control Trial or RCT, where the criteria of inclusion/exclusion are clearly spelled out and there are no lurkers in the mix. The speed of approval is not necessarily a function of OS but the regulatory maze that the drug –maker has to work through. Using OS as the final arbiter may weed out some “would be” contenders early, but maybe you wouldn’t want that when bazillion$ are at stake. This then makes for a speedy review, but at what cost? And that can be bad.
I mean even If there is a bias of tumor biology, the over all survival or OS gives a pretty good indicator of the value of therapy in a well-constructed time-honored validated methodology of a Controlled Trial, don't you think?. If patients live longer and not by a few days or a couple of weeks but by a “significant” number of months or years, the “Evidence” becomes a tad bit stronger about the efficacy of the therapy, medicine can then base its decision on that, rather than the EBM they continue to tout without a shred of understanding. Therein lies the dilemma of why investigational studies fail in the real world. They just do.
Have I been too harsh?
Have I been too unkind?
I must be cruel only to be kind;
Thus bad begins, and worse remains behind ~ Shakespeare






No comments:
Post a Comment